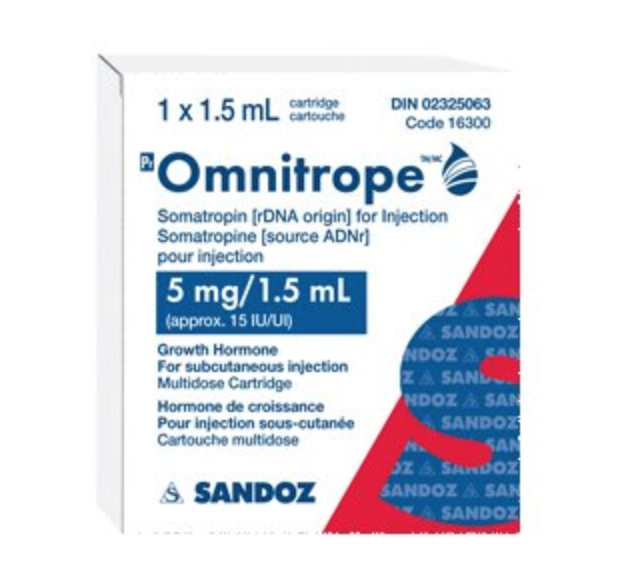
OMNITROPE HGH 15IU
$158.00 $142.00
Manufacturer: Sandoz
Substance: Somatropin
Dosage: 15iu
In Stock
DESCRIPTION
Like other human growth hormone brands, Omnitrope Hgh is a synthetic form of growth hormone that occurs naturally in the human body. Human growth hormone is a protein that stimulates bones and cartilage to grow. Omnitrope Hgh is highly similar in structure to naturally occurring human growth hormone, but it is produced in a laboratory and meets strict standards of product quality. Omnitrope is an FDA-approved product that is proven to help children grow. Omnitrope is also easy to use and can cost less than other treatments.
Omnitrope® indications and important safety information
Omnitrope Hgh is a recombinant human growth hormone indicated for:
- Pediatric: Treatment of children with growth failure due to growth hormone deficiency (GHD), Prader-Willi Syndrome, Small for Gestational Age, Turner syndrome, and Idiopathic Short Stature.
- Adult: Treatment of adults with either adult onset or childhood-onset GHD.
Contraindications
- Acute Critical Illness
- Children with Prader-Willi Syndrome who are severely obese or have severe respiratory impairment—reports of sudden death
- Active Malignancy
- Active Proliferative or Severe Non-Proliferative Diabetic Retinopathy
- Children with closed epiphyses
- Known hypersensitivity to somatropin or excipients
Warnings and Precautions
- Acute Critical Illness: Potential benefit of treatment continuation should be weighed against the potential risk
- Prader-Willi Syndrome in children: Evaluate for signs of upper airway obstruction and sleep apnea before initiation of treatment. Discontinue treatment if these signs occur.
- Neoplasm: Monitor patients with preexisting tumors for progression or recurrence. Increased risk of a second neoplasm in childhood cancer survivors treated with somatropin–in particular meningiomas in patients treated with radiation to the head for their first neoplasm
- Impaired Glucose Tolerance and Diabetes Mellitus: May be unmasked. Periodically monitor glucose levels in all patients. Doses of concurrent antihyperglycemic drugs in diabetics may require adjustment
- Intracranial Hypertension: Exclude preexisting papilledema. May develop and is usually reversible after discontinuation or dose reduction.
- Fluid Retention (i.e., edema, arthralgia, carpal tunnel syndrome, especially in adults): May occur frequently. Reduce dose as necessary
- Hypopituitarism: Closely monitor other hormone replacement therapies
- Hypothyroidism: May first become evident or worsen
- Slipped Capital Femoral Epiphysis: May develop. Evaluate children with the onset of a limp or hip/knee pain
- Progression of Preexisting Scoliosis: May develop
- Otitis Media and Cardiovascular Disorders in Turner syndrome: Patients with Turner syndrome should be evaluated for otitis media and other ear disorders and monitored for cardiovascular disorders• Pancreatitis: Consider pancreatitis in patients with persistent severe abdominal pain, especially children
- Pancreatitis: Consider pancreatitis in patients with persistent severe abdominal pain, especially children
- Adverse events and death associated with benzyl alcohol: Formulations containing benzyl alcohol (5 mg/1.5 mL Omnitrope Cartridges and the Bacteriostatic Water for Injection diluent for the Omnitrope 5.8 mg/vial) should not be used in premature babies or neonates. Consider the combined daily metabolic load of benzyl alcohol from all sources
Adverse Reactions
Other common somatropin-related adverse reactions include injection site reactions/rashes and lipoatrophy and headaches.
Drug Interactions
- 11β-Hydroxysteroid Dehydrogenase Type 1: May require the initiation of glucocorticoid replacement therapy. Patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance doses
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Should be carefully adjusted
- Cytochrome P450-Metabolized Drugs: Monitor carefully if used with somatropin
- Oral Estrogen: Larger doses of somatropin may be required in women
- Insulin and/or Oral Hypoglycemic Agents: May require adjustment
Use In Specific Populations
Pregnancy — Pregnancy Category B
Animal reproduction studies have not been conducted with Omnitrope. It is not known whether Omnitrope can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity.
Nursing Mothers
It is not known whether Omnitrope Hgh is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Omnitrope is administered to a nursing woman
Geriatric Use
The safety and effectiveness of Omnitrope in patients aged 65 and over have not been evaluated in clinical studies. Elderly patients may be more sensitive to the action of somatropin, and therefore may be more prone to develop adverse reactions. A lower starting dose and smaller dose increments should be considered for older patients.
2 reviews for OMNITROPE HGH 15IU
Add a review Cancel reply
Related products
DRAGON PHARMA
In Stock
DRAGON PHARMA
In Stock
MEDICAL PHARMA
In Stock
DRAGON PHARMA
In Stock
BRITISH DRAGON
In Stock
BRITISH DRAGON
In Stock
DRAGON PHARMA
In Stock
DRAGON PHARMA
In Stock







Douglas Nath –
Just to advise items arrived, super fast service, Thanks for the great service and fantastic products.
Dennis Tyler –
Thanks…for the quick response and excellent service